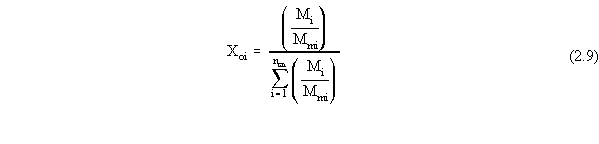
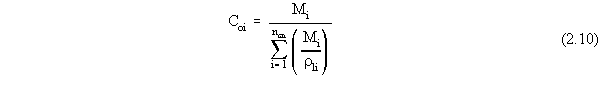
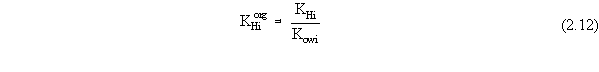
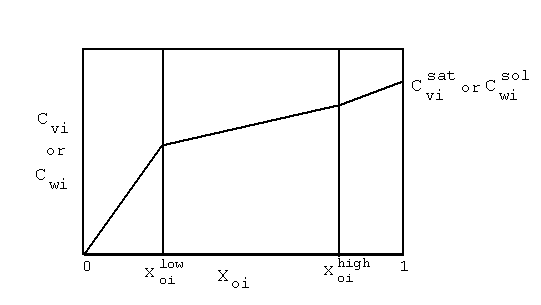
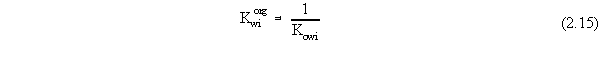
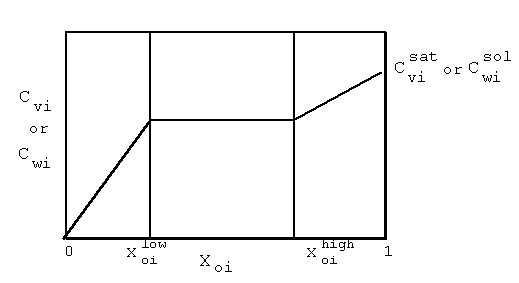
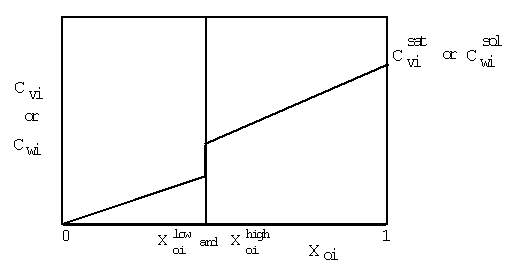
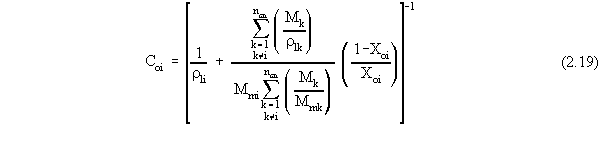
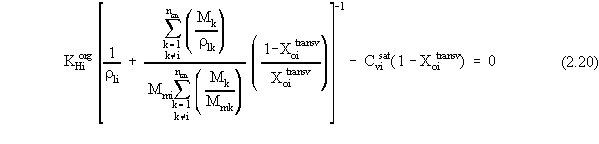
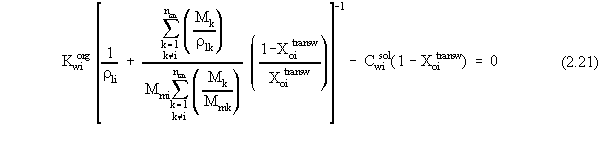
| (a) | Note that in Equations 2.17 and 2.18, the variables with 'trans· ' in their superscript represent the low transition mole fractions or concentration. The high transition mole fractions are equal to one minus those values of the assumption of equally wide regions. |