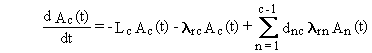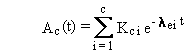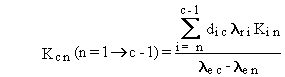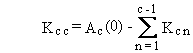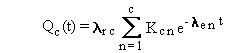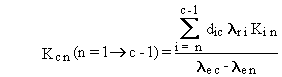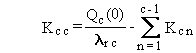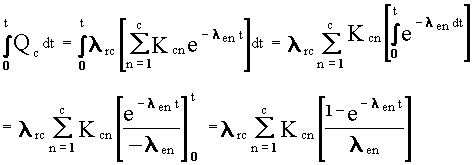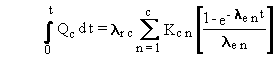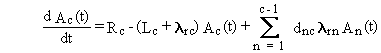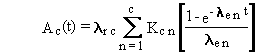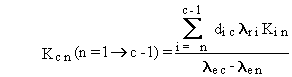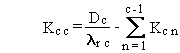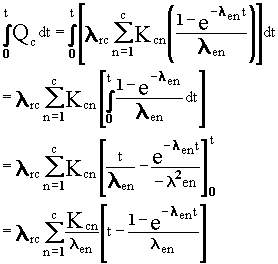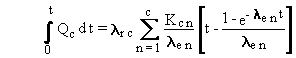APPENDIX B
RADIONCULIDE DECAY ALGORITHMS
A general solution to first-order compartmental models
is presented in this appendix for application to systems consisting of
one physical medium that contains any number of radionuclide decay chain
members. The solution can be applied to any such system involving physical
transfers from the medium and radioactive chain decay with branching. The
general analytical solution to the problem is described mathematically.
The general analytical solution is extended to evaluation of the time integral
of the radionuclide quantities and to cases involving deposition from outside
sources. For deposition at a constant rate during a time period, the general
solution can be applied to determine the quantity present during the time
period and the time integral of the quantity during the time period.
Various methods have been described for evaluating
systems involving radioactive decay (Bateman 1910; Friedlander and Kennedy
1955; Hamawi 1971; Scherpelz and Desrosiers 1980) and physical transfers
between media (Gear 1971; Skrable et al. 1974; Hindmarsh 1983; Birchall
and James 1989; Kirchner 1990). Some of these methods involve simple analytical
solutions, such as the Bateman (1910) representation of the radioactive
decay process without branching; others involve advanced numerical methods
to solve multi-compartment system such as the numerical differential equation
solvers of Gear (1971) and Hindmarsh (1983) and the numerical matrix method
described by Birchall and James (1989). The analytical solutions presented
by Bateman (1910), Scherpelz and Desrosiers (1980), and Skrable et al.
(1974) do not consider branching, but can account for branching by performing
multiple applications of the equations to each possible decay path and
summing the results appropriately, a method suggested by Friedlander and
Kennedy (1955). The general solution presented in this appendix includes
chain decay with branching explicitly in the equations (Kennedy and Strenge
1992).
The general radioactive-decay-chain problem is illustrated
in Figure B.1. In this figure each box represents a radionuclide decay
chain member in a medium. Two types of transfers may be represented: radioactive
decay between chain members and physical transfer from the medium. Radioactive
transitions in this system are represented as flowing from upper boxes
to lower boxes; any upper box may contribute material to any lower box.
Because radioactive transitions within decay chains are irreversible, upward
transfers, representing recycling of material, are not considered. Physical
transfers out of the medium are indicated by the downward arrows from each
box.
This appendix presents four applications of the general
solution for the compartmental system of Figure B.1. First, the solution
is presented for the evaluation of the quantity of radionuclides in each
box as a function of time, based on a user-defined initial inventory. The
general solution is presented
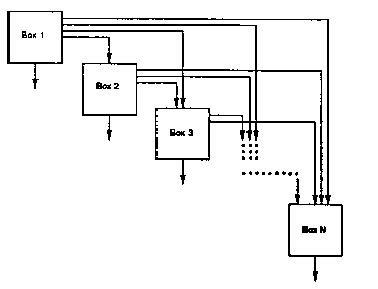
FIGURE B.1 A Representation of the General Radioactive-Decay-Chain Problem
for quantities expressed in units of atoms and activity. The solution
then is extended for use to evaluate three additional situations. The first
extension covers the evaluation of the time integral of the quantity in
each box during a time period. The general solution also is shown to apply
to cases involving deposition of radionuclides at a constant rate to a
medium when the initial quantity in each box is zero. This application
provides the quantity in each box after accumulation during a time period,
and can be extended to provide the time integral of the quantity of each
chain member from deposition accumulation during a time period.
General Solution
An algorithm for evaluations using the general solution
equations is given to demonstrate translation of the method to computer
applications. In the system of boxes as shown in Figure B.1, each box may
involve 1) transfer to any other box lower in the system and 2) loss by
radioactive decay within each box with generation of progeny in a lower
box. Transfers between boxes are described by rate constants. The general
differential equation for the change in the quantity of a radionuclide
in the medium is described by the following word equation:
(Rate of change of chain member c) =
- (rate of physical transfer of chain member c out of the medium)
- (rate of radioactive-transition loss of chain member c)
+ (rate of radioactive-transition ingrowth of chain member c).
Radioactive transitions of precursor radionuclides are represented in the last term.
The equivalent mathematical form of this equation is as follows.
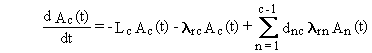
(B.1)
where
Lc = total rate constant for all physical transfers of chain member c from the medium (d-1)
Ac(t) = quantity of chain member c at time t (atoms)
lrc = radioactive transition rate constant for chain member c (d-1)
An(t) = quantity of chain member n at time t (atoms)
dnc = fraction of precursor radionuclide transitions (chain member n) that result in production of the chain member c (dimensionless)
lrn = radioactive transition rate constant for chain member n (d-1).
The first term on the right side of Equation (B.1) represents
physical transfers of chain member c out of the medium. The rate constant,
Lc, is the sum of all physical transfer rate constants from
the media (for chain member c). The second term represents loss by radioactive
transitions of the radionuclide of chain member c to progeny radionuclides
in other boxes. The last term represents production of the chain member
c from all precursor radionuclides.
The general solution can be summarized by the following four equations.
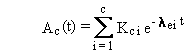
(B.2)
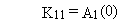
(B.3)
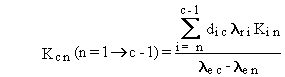
(B.4)
and
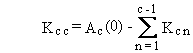
(B.5)
The previous discussion and equations describe quantities
of radionuclides expressed in units of atoms. Equations (B.2) through (B.5)
can be easily converted to units of activity, such as Bq or Ci, using the
general relationship between atom and activity units:
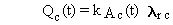
(B.6)
where
Qc(t) = activity of chain member c at time t (activity units)
k = constant of proportionality between activity units and atoms (activity?time/atom)
lrc = radioactive-transition rate constant (inverse time)
and Ac(t) is as previously defined. When activity is expressed
in Bq and time in seconds, the constant equals 1.
Substituting the expression in Equation (B.6) into
Equations (B.2) through (B.5), with the terms slightly simplified, results
in the following general solution with quantities expressed in activity
units:
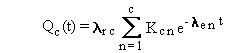
(B.7)
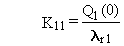
(B.8)
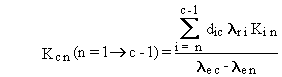
(B.9)
and
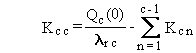
(B.10)
The forms of Equations (B.7) through (B.10) suggest
some limitations on definition of numerical values for rate constants.
First, all boxes must represent a radioactive material, because the radioactive-transition
rate constant appears in the denominator of Equations (B.8) and (B.10).
Stable elements at the end of a decay chain can be simulated as a material
with a long but finite radioactive half-life. This limitation does not
apply to the general solution expressed in atom units, Equations (B.2)
through (B.5), although a stable progeny will effectively terminate a radioactive
decay chain, because the rate constant for a stable isotope is zero. Another
limitation is that the effective rate constant for any two boxes, lec
and len, must not be equal, because
their difference appears in the denominator of Equations (B.4) and (B.9).
This limitation applies only to pairs of chain members that have radioactive
transfers from one to the other.
Use of the general solution given here requires definition
of all rate constants and branching fractions. Data on radionuclide half-lives,
decay chains, and fractional branching within chains has been published
by Lederer and Shirley (1978) and the International Commission on Radiological
Protection in ICRP Publication 38 (ICRP 1983).
Extension to Time-Integration
The discussions and equations to this point have
centered on evaluation of the quantity of radionuclides present as a function
of time. The general solution can be extended easily to provide the time
integral of the quantity present during a time period. This extension is
demonstrated by observing that the general solution includes the time variable,
t, only in the exponential term of Equations (B.2) and (B.7). Obtaining
the time-integral expression involves simply integrating the exponential
expression and evaluating the integral from time zero to the desired time.
The following sequence applied to Equation (B.7) illustrates these steps:
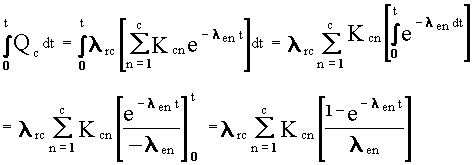
(B.11)
The general solution for the time integral now uses the following formula
with Equations (B.8), (B.9), and (B.10):
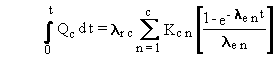
(B.12)
Extension to Deposition at a Constant Rate
Another extension of the general solution applies
to deposition of radionuclides to a medium and accumulation during a time
period. The extension assumes that there are initially no radionuclides
in the medium. The differential equation for chain member c, is based on
Equation (B.1), with an added term representing the constant rate of deposition
of chain member c to the medium, Rc.
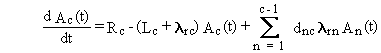
(B.13)
where Rc = constant deposition rate of chain member c to the medium (atoms/d)
and other terms are as previously defined. The general solution to this problem is written as follows, with quantities expressed in activity units:
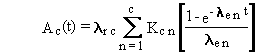
(B.14)
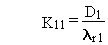
(B.15)
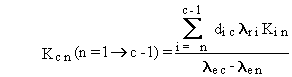
(B.16)
and
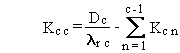
(B.17)
where Dc is the constant deposition rate of chain member
c expressed in terms of activity (pCi/d), and other terms are as previously
defined.
This solution is identical to that for the time-integral
problem except for substitution of Dc for Qc(0),
and the integral of the exponential for the exponential as illustrated
in Equations (B.11) and (B.12).
Extension to Deposition with Time-Integral
The equations for deposition at a constant rate with
accumulation can be integrated to give the time integral of the quantities
in each box during a time period. This integration, similar to that described
in Equation (B.11), works as follows:
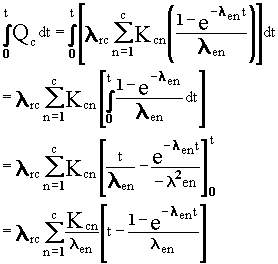
(B.18)
or
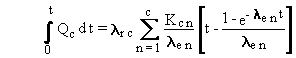
(B.19)
The general solution to the time integral of deposition
at a constant rate with accumulation uses Equation (B.19) [in place of
Equation (B.14)], and Equations (B.15), (B.16), and (B.17). These equations
can be put in terms of atom units by using Equation (B.6), as illustrated
earlier.
REFERENCES
Bateman, H. 1910. "Solution of a System of Differential Equations Occurring
in The Theory of Radio-Active Transformation." Proc. Cambridge Phil.
Soc. 15:423-427.
Birchall, A., and A. C. James. 1989. "A Microcomputer Algorithm for
Solving First Order Compartmental Models Involving Recycling." Health
Phy. 56(6):857-868.
Friedlander, G., and J. W. Kennedy. 1955. Nuclear and Radiochemistry.
John Wiley & Sons, New York.
Gear, C. W. 1971. Numerical Initial Value Problems in Ordinary Differential
Equations, pp 158-166. Prentice-Hall, Englewood Cliffs, New Jersey.
Hamawi, J. M. 1971. "A Useful Recurrence Formula for the Equations of
Radioactive Decay." Nucl. Tech. 11:84-88.
Hindmarsh, A. C. 1983. "Toward a Systemized Collection of ODE Solvers."
Scientific Computing, ed. R. S. Stepleman. 10th IMACS World Congress
on Systems Simulation and Scientific Computation, North-Holland Publishing
Company, Amsterdam, The Netherlands.
ICRP (International Commission on Radiological Protection). 1983. Radionuclide
Transformations - Energy and Intensity of Emissions, Vol. 11-13, ICRP
Publication 38, Pergamon Press, New York.
Kennedy, W. E., Jr., and D. L. Strenge. 1992. Residual Radioactive
Contamination from Decommissioning: Technical Basis for Translating Contamination
Levels to Annual Total Effective Dose Equivalent, Vol. 1. NUREG/CR-5512,
(PNL-7994), U.S. Nuclear Regulatory Commission, Washington, D.C.
Kirchner, T. B. 1990. TIME-ZERO The Integrated Modeling Environment:
Reference Manual. Quaternary Software, Inc., Fort Collins, Colorado.
Lederer, C. M., and V. S. Shirley. 1978. Table of Isotopes, 7th
ed. John Wiley & Sons, Inc., New York.
Scherpelz, R. Il, and A. E. Desrosiers. 1980. "A Modification to a Recurrence
Formula for Linear First-Order Equations." Health Phy. 40:905-907.
Skrable, K., C. French., G. Chabot, and A. Hajor. 1974. "A General Equation
for the Kinetics of Linear First Order Phenomena and Suggested Applications."
Health Phy. 27:155-57.